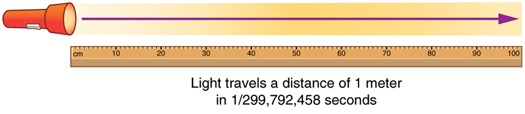
[OpenStax, College physics 2e, Introduction: the nature of science and physics] The nature of science and physics: Physics: an introduction
 Physical quantities and units
Physical quantities and units
The range of objects and phenomena studied in physics is immense. From the incredibly short lifetime of an atomic nucleus to the age of the Earth, from the tiny sizes of sub-nuclear particles to the vast distance to the edges of the known universe, from the force exerted by a jumping flea to the force between Earth and the Sun, there are enough factors of #10# to challenge the imagination of even the most experienced scientist. Giving numerical values for physical quantities and equations for physical principles allows us to understand nature much more deeply than does qualitative description alone. To comprehend these vast ranges, we must also have accepted units in which to express them. And we shall find that (even in the potentially mundane discussion of #\blue{\text{meters}}#, #\green{\text{kilograms}}#, and #\orange{\text{seconds}}#) a profound simplicity of nature appears: all physical quantities can be expressed as combinations of only four fundamental physical quantities: length, mass, time, and electric current.
We define a physical quantity either by specifying how it is measured or by stating how it is calculated from other measurements. For example, we define distance and time by specifying methods for measuring them, whereas we define average speed by stating that it is calculated as distance traveled divided by time of travel.
When we measure a physical quantity, we need to have something to express that measurement, which we do through units.
Measurements of physical quantities are expressed in terms of units, which are standardized values. For example, the length of a race, which is a physical quantity, can be expressed in units of #\blue{\text{meters}}# (for sprinters) or kilometers (for distance runners). Without standardized units, it would be extremely difficult for scientists to express and compare measured values in a meaningful way. There are two major systems of units used in the world: SI units (also known as the metric system) and English units (also known as the customary or imperial system). We will only use the former throughout this text.
Table 1 gives the fundamental SI units that are used throughout this textbook.
| Length | Mass | Time | Electric current |
| #\blue{\text{meter } (\unit{\hspace{-5 mu}}{m})}# | #\green{\text{kilogram }(\unit{\hspace{-5 mu}}{kg})}# | #\orange{\text{second } (\unit{\hspace{-5 mu}}{s})}# | ampere (#\unit{\hspace{-5 mu}}{A}#) |
It is an intriguing fact that some physical quantities are more fundamental than others and that the most fundamental physical quantities can be defined only in terms of the procedure used to measure them.
The units in which those most fundamental physical quantities are measured are thus called fundamental units. In this textbook, the fundamental physical quantities are taken to be length, mass, time, and electric current. All other physical quantities, such as force and electric charge, can be expressed as algebraic combinations of length, mass, time, and current (for example, speed is length divided by time); these units are called derived units.
Below, we discuss the units of time, length, and mass: respectively the #\orange{\text{second}}#, the #\blue{\text{meter}}#, and the #\green{\text{kilogram}}#.
The SI units for time, length, and mass are, respectively, the second, the meter, and the kilogram. Over the years, their definitions have changed in order to make measurements of their associated quantities more accurate. For instance, the current definition of the #\orange{\text{second}}# makes reference to regular atomic vibrations, but it was not always like this. The reason for updating the definitions of these fundamental units is that all measurements are ultimately expressed in terms of fundamental units and can be no more accurate than are the fundamental units themselves.
On the expandable boxes below, you can find a brief history of the many definitions of the #\orange{\text{second}}#, the #\blue{\text{meter}}#, and the #\green{\text{kilogram}}#.
Electric current and its accompanying unit, the ampere, will not be discussed at this point in the course, as the initial modules are only concerned with mechanics, fluids, heat, and waves. In these subjects all pertinent physical quantities can be expressed in terms of the fundamental units of length, mass, and time.
Let us now discuss the metric system.
SI units are part of the metric system. The metric system is convenient for scientific and engineering calculations because the units are categorized by factors of #10#. Table 2 gives metric prefixes and symbols used to denote various factors of #10#.
Metric systems have the advantage that conversions of units involve only powers of #10#. There are #100# centimeters in a #\blue{\text{meter}}#, #1000 \blue{\text{ meters}}# in a kilometer, and so on. In nonmetric systems, such as the system of U.S. customary units, the relationships are not as simple; for instance, there are #12# inches in a foot, #5280# feet in a mile, and so on. Another advantage of the metric system is that the same unit can be used over extremely large ranges of values simply by using an appropriate metric prefix. For example, distances in #\blue{\text{meters}}# are suitable in construction, while distances in kilometers are appropriate for air travel, and the tiny measure of nanometers are convenient in optical design. With the metric system there is no need to invent new units for particular applications.
The powers of #10# discussed above not only make conversions simpler and uniform, but also lead to the very important concept of order of magnitude.
The term order of magnitude refers to the scale of a value expressed in the metric system. Each power of #10# in the metric system represents a different order of magnitude. For example, #{10}^{1}#, #{10}^{2}#, #{10}^{3}#, and so forth are all different orders of magnitude. All quantities that can be expressed as a product of a specific power of #10# are said to be of the same order of magnitude. For example, the number #800# can be written as #8\cdot {10}^{2}#, and the number #450# can be written as #4.5\cdot{10}^{2}#. Thus, the numbers #800# and #450# are of the same order of magnitude: #{10}^{2}#. Order of magnitude can be thought of as a ballpark estimate for the scale of a value. The diameter of an atom is on the order of #{10}^{-9}\blue{\unit{}{ m}}#, while the diameter of the Sun is on the order of #{10}^{9}\blue{\unit{}{m}}#.
At the end of the page you can find Table 3, containing the prefixes for different powers of #10#, their symbols, and some examples of those orders of magnitude.
It is often necessary to convert from one type of unit to another. For example, if you are reading a European cookbook, some quantities may be expressed in units of liters and you need to convert them to cups. Or, perhaps you are reading walking directions from one location to another and you are interested in how many kilometers you will be walking. In this case, you might need to convert units of #\blue{\text{meters}}# to kilometers.
Let us consider a simple example of how to convert units. Let us say that we want to convert #80\blue{\text{ meters}}# (#\unit{\hspace{-5 mu}}{\blue{m}}#) to kilometers (#\unit{\hspace{-5 mu}}{km}#).
The first thing to do is to list the units that you have and the units that you want to convert to. In this case, we have units in #\blue{\text{meters}}# and we want to convert to kilometers.
Next, we need to determine a conversion factor 1000 relating #\blue{\text{meters}}# to kilometers. A conversion factor is a ratio expressing how many of one unit are equal to another unit. For example, there are #100# centimeters in #1\blue{\text{ meter}}#, #12# inches in #1# foot, #60 \orange{\text{ seconds}}# in #1# minute, and so on. In this case, we know that there are #\blue{\text{ meters}}# in #1# kilometer.
Now we can set up our unit conversion. We will write the units that we have and then multiply them by the conversion factor so that the units cancel out, as shown:
\[ \begin{array}{rcl} 80\blue{\unit{}{m}}\cdot\dfrac{\unit{1}{km}}{1000\blue{\unit{}{m}}}&=&\unit{0.080}{km}\end{array} \]
Note that the unwanted#\blue{\unit{}{m}}# unit cancels, leaving only the desired #\unit{\!}{km}# unit. You can use this method to convert between any types of unit.
The tab below gives an impression of the vast range of scales in which physics applies.
Next, you can find an example of unit conversion in a problem. Below that, you can find a table with all the metric prefixes, their values, and some examples of their use.
Unit conversions: a short drive home
Suppose you drive the #\unit{10.0}{km}# from your university to home in #\unit{20.0}{min}#. Calculate your average speed (a) in kilometers per hour (#\unit{\hspace{-5 mu}}{km/h}#) and (b) in meters per second (#\unit{\hspace{-5 mu}}{\blue{m}/\orange{s}}#).
Note: average speed is distance traveled divided by time of travel.
1. Outline a strategy
First we calculate the average speed using the given units. Then we can get the average speed into the desired units by picking the correct conversion factor and multiplying by it. The correct conversion factor is the one that cancels the unwanted unit and leaves the desired unit in its place.
2. Solve for speed (a)
- Calculate average speed. Average speed is distance traveled divided by time of travel. Take this definition as a given for now, average speed and other motion concepts will be covered in a later module. In equation form this definition reads
\[ \text{average speed} =\dfrac{\text{distance}}{\text{time}}\] - Substitute the given values for distance and time.
\[ \text{average speed} =\dfrac{\unit{10.0}{km}}{\unit{20.0}{min}}=\unit{0.500}{km/min}\] - Convert#\unit{}{km/min}# to#\unit{}{km/h}#: multiply by the conversion factor that will cancel minutes and leave hours. There are #60# minutes in each #1# hour. The conversion factor is therefore
\[\dfrac{\unit{60}{min}}{\unit{1}{h}} = \unit{60}{\dfrac{min}{h}}\] which we can use to obtain the answer
\[ \begin{array}{rcl}\text{average speed} &=& \unit{0.500}{\dfrac{km}{min}}\cdot \unit{60}{\dfrac{min}{h}}\\ &=&\unit{30.0}{\dfrac{km}{h}}\end{array}\]
Discussion
To check your answer, consider the following steps.
- Be sure that you have properly canceled the units in the unit conversion. If you have written the unit conversion factor the other way around, the units will not cancel properly in the equation; rather, they will give you the wrong units as follows:
\[\dfrac{\unit{\!}{ km}}{\unit{\!}{min}}\cdot \dfrac{\unit{1}{ h}}{\unit{60}{ min}}=\dfrac{1}{60}\dfrac{\unit{\!}{ km\cdot h}}{\unit{\!}{ min^2}} \]
which are obviously not the desired units of#\unit{}{km/h}#. - Check that the units of the final answer are the desired units. The problem asked us to solve for average speed in units of #\unit{\!}{km/h}# and we have indeed obtained these units.
- Check the significant figures. Because each of the values given in the problem has three significant figures, the answer should also have three significant figures. The answer #\unit{30.0}{km/h}# does indeed have three significant figures, so this is appropriate. Note that the significant figures in the conversion factor are not relevant because an hour is defined to be #60# minutes, so the precision of the conversion factor is perfect.
- Next, check whether the answer is reasonable. Let us consider some information from the problem: if you travel #\unit{10}{km}# in a third of an hour (#\unit{20}{min}#), you would travel three times that far in an hour. The answer does seem reasonable.
3. Convert units (b)
There are several ways to convert the average speed into meters per second.
- Start with the answer to (a) and convert #\unit{\!}{km/h}# to #\unit{\!}{\blue{m}/\orange{s}}#. Two conversion factors are needed: one to convert hours to seconds, and another to convert kilometers to meters. Every #1# hour consists of #3600# seconds and there are #1000# meters in each #1# kilometer. The resulting conversion factors are therefore: \[ \dfrac{\unit{1}{h}}{3600\orange{\unit{}{s}}} \qquad \text{and} \qquad \dfrac{1000\blue{\unit{}{m}}}{\unit{1}{km}}\]
- Multiplying by these yields
\[ \begin{array}{rcl}\text{Average speed}&=&\unit{30.0}{\dfrac{ km}{ h}} \cdot \dfrac{\unit{1}{ h}}{3600\orange{\unit{}{s}}} \cdot \dfrac{1000\blue{\unit{}{ m}}}{\unit{1}{km}}\\ &=& \unit{8.33}{\dfrac{\blue{m}}{\orange{s}}}\end{array}\]
Discussion
If we had started with #\unit{0.500}{km/min}#, we would have needed different conversion factors, but the answer would have been the same: #\unit{8.33}{\blue{m}/\orange{s}}#.
| Prefix | Symbol | Value | Example (some are approximate) | |||
| exa | E | #{10}^{18}# | exameter | Em | #\unit{{10}^{18}}{m}# | distance light travels in a century |
| peta | P | #{10}^{15}# | petasecond | Ps | #\unit{{10}^{15}}{s}# | 30 million years |
| tera | T | #{10}^{12}# | terawatt | TW | #\unit{{10}^{12}}{W}# | powerful laser output |
| giga | G | #{10}^{9}# | gigahertz | GHz | #\unit{{10}^{9}}{Hz}# | a microwave frequency |
| mega | M | #{10}^{6}# | megacurie | MCi | #\unit{{10}^{6}}{Ci}# | high radioactivity |
| kilo | k | #{10}^{3}# | kilometer | km | #\unit{{10}^{3}}{m}# | about 6/10 mile |
| hecto | h | #{10}^{2}# | hectoliter | hL | #\unit{{10}^{2}}{L}# | 26 gallons |
| deka | da | #{10}^{1}# | dekagram | dag | #\unit{{10}^{1}}{g}# | teaspoon of butter |
| — | — | #{10}^{0}# (=1) | ||||
| deci | d | #{10}^{-1}# | deciliter | dL | #\unit{{10}^{-1}}{L}# | less than half a soda |
| centi | c | #{10}^{-2}# | centimeter | cm | #\unit{{10}^{-2}}{m}# | fingertip thickness |
| milli | m | #{10}^{-3}# | millimeter | mm | #\unit{{10}^{-3}}{m}# | flea at its shoulders |
| micro | µ | #{10}^{-6}# | micrometer | µm | #\unit{{10}^{-6}}{m}# | detail in microscope |
| nano | n | #{10}^{-9}# | nanogram | ng | #\unit{{10}^{-9}}{g}# | small speck of dust |
| pico | p | #{10}^{-12}# | picofarad | pF | #\unit{{10}^{-12}}{F}# | small capacitor in radio |
| femto | f | #{10}^{-15}# | femtometer | fm | #\unit{{10}^{-15}}{m}# | size of a proton |
| atto | a | #{10}^{-18}# | attosecond | as | #\unit{{10}^{-18}}{s}# | time light crosses an atom |

Or visit omptest.org if jou are taking an OMPT exam.